At the heart of the ImmunoSep project was the clinical study conducted with 280 patients with sepsis. The study, designed as a randomized controlled trial, took place over nearly three years across five European countries: Greece, the Netherlands, Germany, Switzerland, and Romania.
The study aimed to:
- test the feasibility of a personalised immunotherapy approach in patients with sepsis guided by host response biomarkers
- assess whether personalised immunotherapies, that can revert either Macrophage activation-like syndrome (MALS) or immunoparalysis, have a beneficial impact on the outcome of patients with sepsis
The ImmunoSep study greatly benefited from insights generated by the PROVIDE project for its design and conduct. This includes how to set-up the diagnostic laboratory platform for a trial of precision medicine based immunotherapy, how to assign enrolled patients into treatment arms and how to feedback assignment in a blind fashion to the investigators, and how to conduct pharmacovigilance.
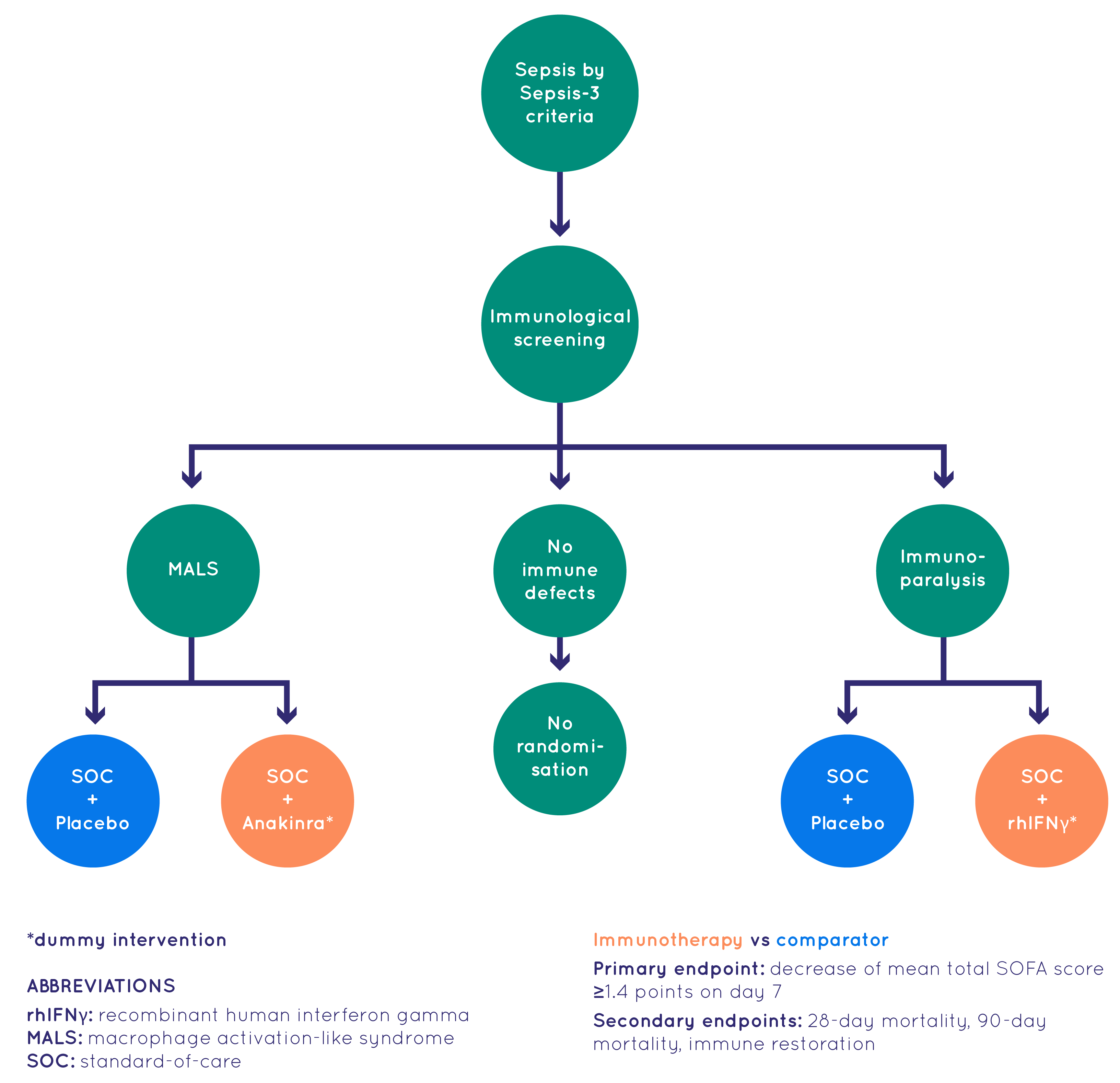
Patients included in the ImmunoSep trial were adults of both genders with sepsis and with community-acquired or healthcare-associated lower respiratory tract infections or primary bacteraemia (bacteria in the bloodstream) for whom written informed consent was provided by a first-degree relative.
Participants were assigned to two arms of treatment during the trial:
- standard-of-care arm receiving intravenous placebo three times daily and subcutaneous placebo once every other day for 15 days;
- personalised immunotherapy arm receiving intravenous 200mg anakinra three times daily and subcutaneous placebo once every other day for 15 days or intravenous placebo three times daily and subcutaneous rhIFNγ once every other day for 15 days depending on screening findings (MALS or immunoparalysis respectively).
The off-target immunological effects of both anakinra (rIL-1Ra) and immukine (rIFN) are well known, and very rarely lead to the discontinuation of the treatment. The investigators have extensive experience with these medications. Results of the phase II study PROVIDE have validated that patients with sepsis are classified to one of three immune endotypes; MALS, sepsis-induced immunoparalysis and unclassified. Patients with MALS were randomized to 7-day treatment with placebo or anakinra. Clinical benefit associated with survival and decrease of SOFA score was found at the end-of-treatment in the anakinra arm have been currently observed in the ongoing PROVIDE sepsis trial.